Application Spotlight: 3D Printed Hearing Aids
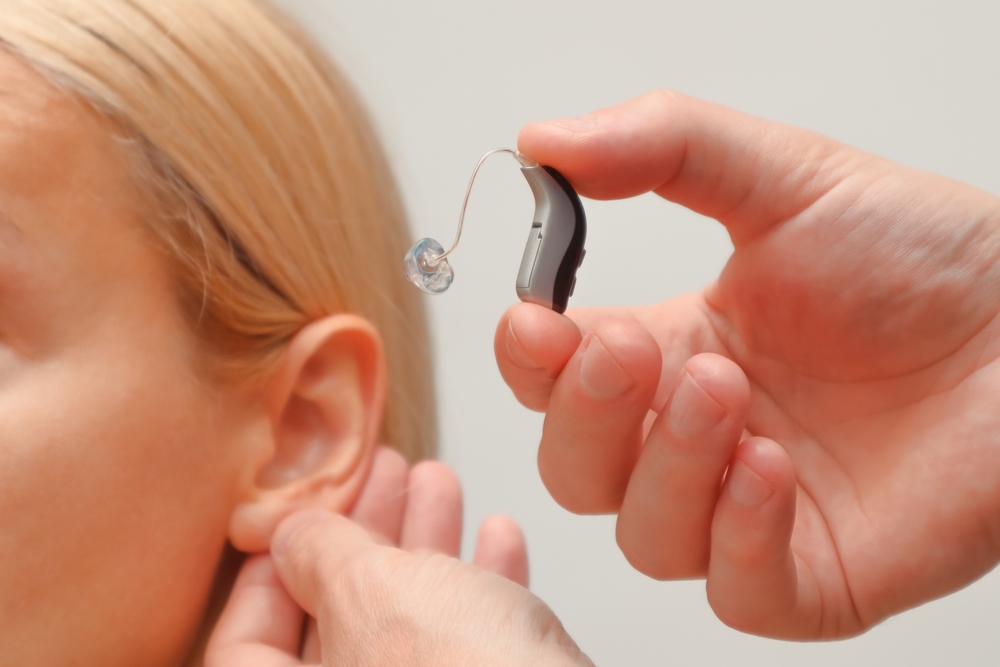
3D printing has revolutionised hearing aid production.Healthcare applications have paved the way for AM technology being used in high volume production lines.3D printing has been touted as the future for customised, distributive manufacturing, but adoption to end-use contexts has experienced a slower journey– except in healthcare.AM’s inclination towards customisation and the speed at which products can be designed and produced has spelt a boom for the production of 3D printed healthcare devices, including implants, orthotics and prosthetics, dental parts, medical instruments, realistic functional prototypes, and, in particular, hearing aids.Early innovators Sonova implemented 3D printing processes for hearing aids at the beginning of the millennium, and soon other companies followed suit. By 2007, 3D printing had become an established technology in hearing aid production. The adoption of AM increased trade by around 60% and drove down production costs. This positive trajectory continued through until 2016, in which US imports grew by 77%.Today it’s estimated that nearly all hearing aids are 3D printed. Sonova hold a large share of the market (31% in 2019), a market showing promise for the future– Technavio forecasted an increase of $636.9 million and growth at a CAGR of 22.21% between 2023 and 2028.
Why is 3D printing used for hearing aids?[spacer height="10px"]

Before the advent of 3D printing, the production of hearing aids was an expensive process carried out by modelers who finished each unique piece in a time-consuming and costly process.But AM has opened the doors to the possibility of de novo design, and the capability to tailor to an individual's unique ear shapes and hearing requirements. 3D printing has driven down cost, as manufacturing complex medical devices has always been an expensive process.Similarly, AM has increased time efficiency. Time-to-market is much shorter when 3D printing is leveraged; it is quick to design and produce parts without carrying out intricate processes.
How the tech works[spacer height="10px"]
[caption id="attachment_43871" align="aligncenter" width="800"]

3D printing hearing aid ear molds on an ASIGA PRO 2 3D Printer. Image courtesy of 3DP4ME.[/caption]Shells for custom in-the-ear hearing aids and now various custom earpieces for behind-the-ear and receiver-in-the-canal hearing aids are now additively manufactured. The shape of the shell can be optimally tailored to the wearer’s ear canal and degree of hearing loss, with vastly improved cost efficiency and enhanced productivity.Silicone impressions of the ear canal (which for mass production are still taken by hand) are taken and then scanned via laser technology. After digital processing, the CAD file of the hearing aid shell is stored in a central database and then transmitted to 3D printers at a production site.Then, layers of acrylic resin or silicone are built on top of each other through stereolithography to produce the hearing shell. Following this, shells are serialised and go through post-processing and testing.
Case studies[spacer height="10px"]
The technology is established, and has swiftly developed into the predominant production method for hearing devices. Therefore, innovation in this area has shifted towards creative applications that push the boundaries on what’s possible in the future in healthcare.Here are 3 of those:
3D printed custom ear implant[spacer height="15px"]
[caption id="attachment_43873" align="aligncenter" width="700"]

Image courtesy of Desktop Health[/caption]This innovation from the ENT Clinic at Hannover Medical School at the end of 2023 comprised a functional, bioprinted implant, customised and able to release drugs in an adult patient.The device functions like a stent, meaning it can prevent an ear passage from narrowing, whilst simultaneously supplying the patient with active pharmaceutical ingredients to promote healing. According to ENT Clinic Director Dr. Thomas Lenarz, this innovation has ‘opened the door to a new type of pioneering patient care’.The device, which was produced with the help of the Desktop Health 3D-Bioplotter Manufacturing Series 3D printer, represents an exciting instance of 3D printing breaking into new possibilities, combining bioprinting technology with customisable hearing devices to increase the capabilities of AM in healthcare.
Efficient 3D printing technology for hearing-aid earmolds[spacer height="15px"]
[caption id="attachment_43875" align="aligncenter" width="640"]

Image courtesy of Callier Center for Communication Disorders at The University of Texas at Dallas[/caption]A new lab has enabled efficient 3D printing for hearing-aid earmolds.In August 2024, the Callier Center for Communication Disorders at the The University of Texas opened a clinical innovation lab which utilised 3D printing for on-site earmold creation. The utilisation of AM drastically reduces the time required for fitting a patient’s device.The earmold channels sound from the hearing aid to the eardrum, and each device is custom-made to fit each person’s ear canal. This is particularly pertinent for the treatment of children with hearing problems, as growing young children typically need multiple sets of earmolds in their first five years.Between 2022 and 2024, Callier printed more than 1200 earmolds in-house for more than 800 patients. The typical process of ordering earmolds has been slashed from a matter of two to three weeks to less than six hours.These advances indicate AM’s potential to expand beyond merely producing hearing devices in a more time and cost efficient manner, but to completely revolutionise how we perceive production and time-to-market.
Non-profit company eyes decentralised care[spacer height="15px"]
[caption id="attachment_43879" align="aligncenter" width="640"]
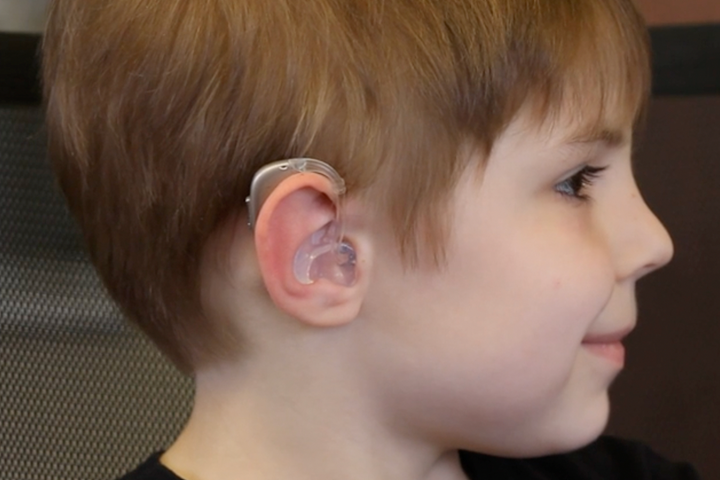
Image courtesy of 3DP4Me[/caption]In 2022, 3DP4ME set the goal of supplying children and adults with devices custom made through a combination of specialised scanning and 3D printing.The technology involves a specialised scanner (provided by Lantos Technologies) which inflates inside the patient’s ear like a balloon, capturing tens of thousands of data points in a matter of seconds. These can then be converted into a 3D printable design for the required earmolds– although this isn’t yet available for younger children.Yet 3DP4ME have their attention primarily not on the technology, but on somewhere else.The challenges that the company are aiming to confront revolve around design, workforce, training, and funding; the technology is ready to be used, but unless care is decentralised then the social impacts of additively manufacturing hearing care cannot be fully realised.3DP4ME have generated initiatives to address these challenges. Mobility is a key challenge, as although the scanner can be entirely mobile, the printer itself cannot. In the future, vans could be sent out to remote locations to scan and then deliver completed devices to patients. Similarly, lack of personnel has to be remedied, as in some countries there are little to no audiologists.3DP4ME’s foray into the decentralisation of care represents a promising step towards AM being seriously considered in real-world applications for the most vulnerable.
Final thoughts[spacer height="10px"]
Demand for 3D printed hearing devices will sky-rocket in the coming years with aging populations in several key markets. In the US, which accounts for the largest share of the hearing aid market, the number of people aged 65+ will more than double over the next 40 years, reaching 80 million in 2040. Japan, another big importer, has one of the oldest populations in the world.Mirrored technological advances in other sectors will continue to shepherd innovation in AM hearing aids. IoT-integrated devices are increasingly being used, with devices linking to smartphones rapidly becoming the norm.3D printing and hearing aid device production are a match made in heaven, but there are still some issues worth considering.The materials needed to produce durable, skin-friendly, high-performance hearing aids are still limited, which can restrict customisation and quality of the final product. Furthermore, the lack of personnel (as experienced by 3DP4ME) presents a challenge.There are regulatory hurdles and stringent health and safety standards to consider which may delay time to market. Manufacturers of 3D printed hearing devices will benefit from MES that ensures these standards are met.AMFG recently secured ISO 27001 certification and allows cutting-edge traceability, so that manufacturers can effortlessly show regulatory compliance to clinicians and authorities.
About AMFG[spacer height="10px"]
AMFG is an award winning MES designed to empower production workflows, from order placement to shipment, with seamless integration and precision automation.With over 500 successful implementations in 35 countries and across a range of industries, we specialize in enabling companies to successfully integrate our software for AM and CNC production, into their wider manufacturing processes and scale their AM operations.To find out more, click here: Book a demo







.svg)